

http://www.selfsufficiencymagazine.com/how-to-treat-migraines-with-red-raspberry-leaf/
If you, or someone close to you, suffers from migraines then you’ll know just how frustrating it can be. You can try all sorts of approaches and conventional medications, but often they don’t work!
Why not try some red raspberry leaf tea? It’s packed full of essential vitamins and minerals and is widely used for helping to cure those painful headaches.

On June 27th, Vertex Pharmaceuticals gets European CHMP’s (Committee for Medicinal Products for Human Use) positive opinion recommending the approval of orphan drug Kalydeco (Ivacaftor) for Cystic Fibrosis (CF) patients, ages 6 and older, who have 1 of the following 8 non-G551D gating mutations, in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene:
•G178R •S549N •S549R •G551S •G1244E •S1251N •S1255P •G1349D.
These 8 additional mutations affects approximately 250 patients in Europe. The next step, is for the European Commission (EC), that has the authority to approve drugs for the European Union, to review the CHMP’s positive opinion. The EC usually follows the recommendation of the CHMP and issues a marketing approval within 3 – 4 months. Kalydeco receives approval in Europe in July 2012 for patients with CF ages 6 and older, who have at least 1 copy of the G551D mutation, which is the most common gating…
View original post 277 more words
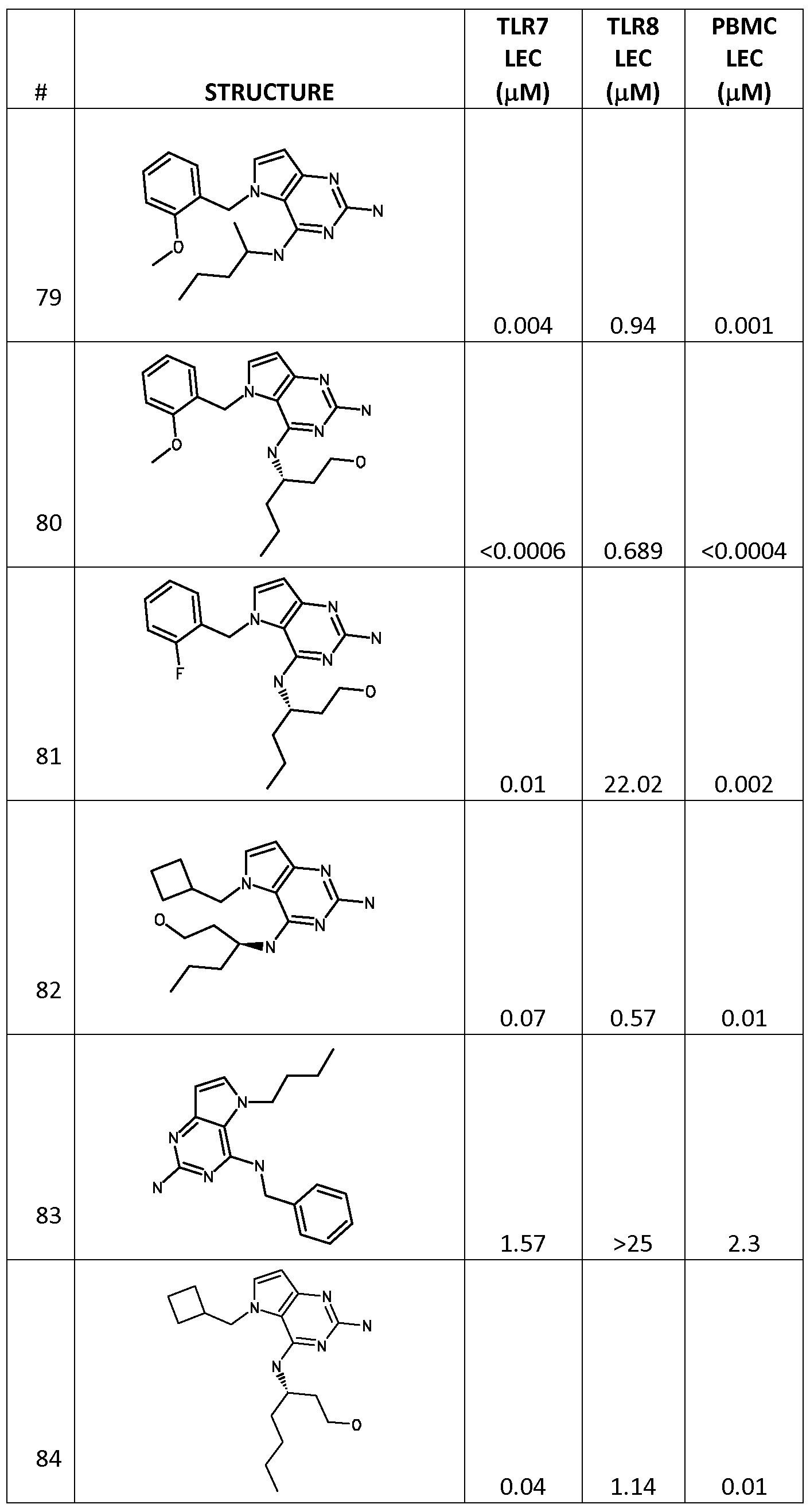
COMPOUND 80 ABOVE
IN
http://www.google.com/patents/WO2014056953A1?cl=en
(3S)-3-[[2-Amino-5-(2-methoxybenzyl)-5H-pyrrolo[3,2-d]pyrimidin-4-yl]amino]heptan-1-ol
TLR7 Receptor Agonists , Signal Transduction Modulators
Janssen R&D Ireland (INNOVATOR)
383.4873
C21 H29 N5 O2
DRUG REGULATORY AFFAIRS INTERNATIONAL
GDP Question: When to use Mean Kinetic Temperature Calculation (MKT)?
The British Medicines Authority MHRA is performing GMP and GDP Inspections on a risk based strategy. Based on the experience the MHRA answers frequent asked questions. One very interesting Q&A refers to Mean Kinetic Temperature Calculations (MKT). Read more about MKT here
DRUG REGULATORY AFFAIRS INTERNATIONAL
How to become a QP for Europe
Both the ECA and the European QP Association are often contacted by people who would like to become a Qualified Person in a Member State of the European Union or outside the EU to release products for the EU market. Read more.
May 8, 2014 — The U.S. Food and Drug Administration today approved Zontivity (vorapaxar) tablets to reduce the risk of heart attack, stroke, cardiovascular death, and need for procedures to restore the blood flow to the heart in patients with a previous heart attack or blockages in the arteries to the legs.
Zontivity is the first in a new class of drug, called a protease-activated receptor-1 (PAR-1) antagonist. It is an anti-platelet agent, designed to decrease the tendency of platelets to clump together to form a blood clot. By decreasing the formation of blood clots, Zontivity decreases the risk of heart attack and stroke.
Like other drugs that inhibit blood clotting, Zontivity increases the risk of bleeding, including life-threatening and fatal bleeding. Bleeding is the most commonly reported adverse reaction in people taking Zontivity. The drug’s prescribing information (label) includes a Boxed Warning to alert health care professionals about this…
View original post 245 more words

MILTEFOSINE
2-(hexadecoxy-oxido-phosphoryl)oxyethyl-trimethyl-azanium
58066-85-6
March 19, 2014 — The U.S. Food and Drug Administration today approved Impavido (miltefosine) to treat a tropical disease called leishmaniasis.
Leishmaniasis is a disease caused by Leishmania, a parasite which is transmitted to humans through sand fly bites. The disease occurs primarily in people who live in the tropics and subtropics. Most U.S. patients acquire leishmaniasis overseas.
Impavido is an oral medicine approved to treat the three main types of leishmaniasis: visceral leishmaniasis (affects internal organs), cutaneous leishmaniasis (affects the skin) and mucosal leishmaniasis (affects the nose and throat). It is intended for patients 12 years of age and older. Impavido is the first FDA-approved drug to treat cutaneous or mucosal leishmaniasis.
“Today’s approval demonstrates the FDA’s commitment to making available therapeutic options to treat tropical diseases,” said…
View original post 2,887 more words
Consumption of cinnamon is associated with favorable reductions in plasma glucose and lipid levels, according to research published in the September/October issue of the Annals of Family Medicine.
Robert W. Allen, Pharm.D., of the Western University of Health Sciences in Pomona, Calif., and colleagues used data from 10 randomized, controlled trials involving 543 patients with type 2 diabetes to conduct an update of a previous systematic review and meta-analysis examining the effect of cinnamon consumption on glucose and lipid levels.
The researchers found that cinnamon, in daily doses of 120 mg/d to 6 g/d for four to 18 weeks, was associated with a significant reduction in levels of fasting plasma glucose (?24.59 mg/dL), but no significant effect on glycosylated hemoglobin. Cinnamon intake also was linked to significant changes in lipid levels, including decreases in levels of total cholesterol (?15.60 mg/dL), low-density lipoprotein cholesterol (LDL-C) (?9.42 mg/dL), and triglycerides…
View original post 89 more words

Panobinostat
HDAC inhibitors, orphan drug
cas 404950-80-7
2E)-N-hydroxy-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]acrylamide
N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide (alternatively, N-hydroxy-3-(4-{[2-(2-methyl-1H-indol-3-yl)-ethylamino]-methyl}-phenyl)-acrylamide)
Molecular Formula: C21H23N3O2 Molecular Weight: 349.42622
- Faridak
- LBH 589
- LBH589
- Panobinostat
- UNII-9647FM7Y3Z
A hydroxamic acid analog histone deacetylase inhibitor from Novartis.
NOVARTIS, innovator
Histone deacetylase inhibitors
Is currently being examined in cutaneous T-cell lymphoma, CML and breast cancer.
clinical trials click here phase 3
DRUG SUBSTANCE–LACTATE AS IN http://www.google.com/patents/US7989639 SEE EG 31

Panobinostat (LBH-589) is an experimental drug developed by Novartis for the treatment of various cancers. It is a hydroxamic acid[1] and acts as a non-selective histone deacetylase inhibitor (HDAC inhibitor).[2]
panobinostat
Panobinostat is a cinnamic hydroxamic acid analogue with potential antineoplastic activity. Panobinostat selectively inhibits histone deacetylase (HDAC), inducing hyperacetylation of core histone proteins, which may result in modulation of cell cycle protein expression, cell cycle arrest in the…
View original post 5,150 more words